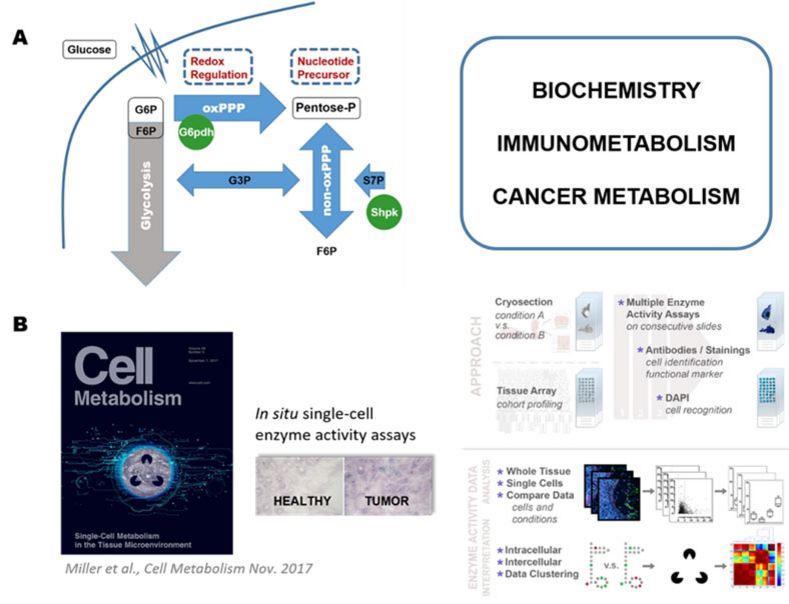
A) We investigate how cellular carbohydrate metabolism, here schematically represented by glycolysis and the pentose phosphate pathway (PPP), regulates cellular physiology. Both pathways are interlinked and these nodes appear to be highly regulated during metabolic reprogramming. The PPP is of special interest, as it controls cellular redox-states and anabolic processes like nucleotide precursor formation. We apply this knowledge to find novel strategies to fight human diseases.
B) A recently published imaging approach to assess enzymatic activities and metabolic profiles of single cells in situ. With this tool we managed to characterize the first metabolic profiles of single human immune cells within intact tissue microenvironments. Additionally, we used this method to assess metabolic circuits of different cell types (intercellular metabolism) in human tumor tissues.
Carbohydrate metabolism and adaptations of cellular functions
Cells need energy and metabolic precursors to fulfill their biological functions. When cells adapt their functions also their metabolic machinery adapts, reflecting altered metabolic demands to sustain new functions. This link between metabolic-states and function is important to understand cellular behavior. This is particularly true for cancer biology and immunology. Both, malignant cell-transformation and immune cell activation result in a significant reprogramming of cellular carbohydrate metabolism and functions. We hypothesize that understanding this interplay and molecular mechanisms in more detail, including cross-talks of metabolic fluxes, protein signaling, transcriptional and epigenetic regulations in cells, will reveal novel therapeutic opportunities to treat cancer and inflammatory diseases in humans or guide clinical decisions making in precision medicine.
Therefore, we aim to characterize metabolic profiles of cells and functionally associate them with physiological and pathophysiological conditions. Key areas of research include the immuno-metabolic mechanism regulating macrophage activation in acute and chronic inflammatory diseases, and the identification of metabolic wiring of cancer and associated cells in the tumor microenvironment of colorectal and pancreatic cancer patients.
New players in cellular metabolism
Carbohydrate metabolism is one the most fundamental aspects of life, since it sustains physiology and is associated with severe human diseases, such as cardiovascular disease and cancer. To tackle these major challenges of medical science and to design efficient therapies, we need to extend our current understanding of the underlying biochemical processes and their physiological consequences. Recently, we and others have identified the first heptose kinase (SHPK, previously known as CARKL) in vertebrates forming sedoheptulose-7-phosphate, a direct intermediates of glucose-dependent biochemical pathways in cells. We characterized this novel carbohydrate kinase as an endogenously triggered metabolic-switch during macrophage polarization, in human and mice, re-routing cellular carbon-flux at the interface of glycolysis and PPP to meet the specific metabolic demands of respective activation states. We are currently investigating the physiological relevance of heptose metabolism in humans and focus on mechanism important for metabolic reprogramming in different human diseases, including the development and progression of atherosclerosis. Moreover, we are pursuing to identify and characterize additional novel metabolic enzymes for providing an updated perspective of mechanism driving and regulating carbohydrate metabolism in cells.
Techniques and infrastructure of the research group
The laboratory has strong expertise in biochemistry, cellular and molecular biology research and is fully equipped for cell and organ culture, cloning, PCR, recombinant protein production/purification, quantitative and qualitative small molecule analysis, biochemical assays, histology, imaging, FACS, various cell-based assays like gene targeting and licenses for various bioinformatics software tools.
Very recently, we purchased the Vectra Polaris Automated Quantitative Pathology Imaging System (Perkin-Elmer), which integrates both multispectral imaging and automated slide scanning to visualize, analyze, quantify, and phenotype single cells in situ. The group develops and utilizes mouse models to study carbohydrate metabolism in vivo. Moreover, the group has an ongoing collaboration with the Department of Surgery to obtain unused primary human material from adult organ donors to investigate carbohydrate metabolism in humans. Group members have access to next generation sequencing equipment and the department’s state-of-the art mass-spectrometry instruments.